- DIY
- A
Homemade amateur radio tubes. France, 1920. Part 1
Obsolete electro-vacuum devices are still used in limited and special cases to this day, not to mention the trend of "tube sound" and digital indicators of glowing discharge. Amateur homemade vacuum tube work also finds its followers — as a technical retro hobby — a very complex and multifaceted activity: it involves vacuum technology, chemistry, physics, electronics, glassblowing, and working with high temperatures and exotic materials. Even the manufacture of a simple, more or less modern, factory-made incandescent bulb involves hundreds of operations, the exact repetition of which is difficult and unnecessary for the hobbyist. In this sense, it is useful to look back, to the origins, to the time when the first simple electro-vacuum devices appeared, including handmade ones, sometimes crafted with modest means and efforts.
The text will discuss the works of the French enthusiast M. N. Minier, several articles of which were published in the technical collection of 1920. A free translation-summary of these, along with my own comments, is presented here for the esteemed public. Imagine — a workshop without the familiar equipment, devices, materials, or even grid electricity. Only the flame of an alcohol lamp, only glass and a little wire, only boundless enthusiasm, inventiveness, and love for radio technology.
I confess, I never imagined in the past that bright light bulbs (probably vacuum ones), which illuminate us and are so easily broken, could be made by human hands... My childlike imagination thought they must be born by themselves, like butterflies, like flowers, like crystals. And it is because of the unexpected and mesmerizing spectacle of this work that we take an interest in it and dedicate much time to it in the dim light of the blowing shop.
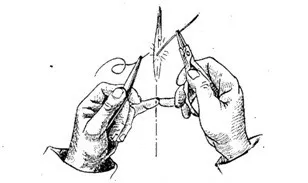
It is worth knowing in advance that working with glass can be somewhat thankless, but not difficult. It happens — after the master has worked for a long time, burned his fingers, spilled gasoline, and overcame all difficulties... a light dry crack, recognizable among a thousand others, saying that the glass work has shattered — now it is lost forever. At the beginning of training, this happens often. We leave the blowtorch in anger, break the cracked work, but return soon, more passionate than ever.
Try working with glass. Even if you don't want to make vacuum bulbs, haven't invented anything, or are rich enough not to have such worries, you might need to make glass joints, fittings, funnels, or even assemble small regular incandescent bulbs that you will proudly light in the respectful eyes of your students and admirers.
Know how to build a vacuum bulb, it will be useful. You are a master and an inventor, you love radio, you also love chemistry and physics, you have been following research in rarefied gases, the effects of cathode radiation, and X-rays — all experiments requiring very low pressure and enclosures made of glass or crystal. You will enjoy watching the transformations of the high-voltage discharge in the lamp, gradually losing air. You will be happy to achieve a vacuum, it's so valuable in our scientific age.
Be patient and don't get upset. Be calm throughout the session, be the master of your nerves. Don't work frantically, learn to be slow and steady. This work is more mentally exhausting than physically. I still know and am capable of too little in this area, but I know that achieving such a result is possible for anyone.
M. N. Minye. 1920.
1. Consumables
First, the most expensive and complicated.
Mercury: I dare not speak of what has been purchased... Like oil, transportation, and taxes, it has become more expensive! I wish for you to have old barometers, thermometers, etc., that can be disassembled without incurring paternal wrath. I will venture to guess: the mercury that will flow in your devices does not wear out: it serves indefinitely without polluting. You could together create an association, a club, one of whose members had acquaintance with a physics cabinet, and the laboratory manager would be happy, in the name of science, to lend you a kilogram or two of mercury. The scales will act as intermediaries and always mark any possible leaks. Otherwise, the minimum amount you would need to find would be just over 400 grams to avoid making the lamp pumping too labor-intensive.
Platinum: very expensive. Look for all (very) old radio tubes, they sometimes contain it. Often, platinum is replaced by "platinite" (a special bimetal), which is useless for you (!) because it requires a certain glass with the same expansion coefficient. The diameter of the platinum wire: 0.2 mm, 0.25 mm, very good 0.3 mm. Buy one meter if you want to have a small stock, but for the first time, a few centimeters may be enough.
Tungsten: the last difficulty. Tungsten can be found for sale in rolls. If you know the manufacturer of the incandescent bulbs, ask him to give you tungsten for a 25-candle (~25 W) bulb (at 110 volts, it passes 0.25 amps). If you want to make the bulbs more reliable, but with a higher filament current, ask for wire for 50 candles (0.5 amps at 110 volts). Do not take tungsten from used bulbs or even new ones — it becomes brittle from the moment it is first turned on. Or look for bulbs with tantalum wire, which does not become brittle even after use.
Easy-to-access consumables:
Aluminum sheet: A small piece 9x12 centimeters and 0.2 mm thick will work very well. From it, we will make lamp electrodes, which are only used for receiving electrons (anodes). This is a good metal, pure and spark-free in a vacuum, and is often preferred by Americans.
Aluminum wire: one meter, Ø 3 mm.
Copper wire: for the internal frame (traverse), for legs and external wires. About 60 cm of Ø 0.4 mm wire is required for each lamp. Ø 0.5 mm is also needed, and for grids Ø 0.3 mm.
Copper wire mesh: For vacuuming (pumping) lamps with heating. Fine-mesh mesh for car fuel filters works very well.
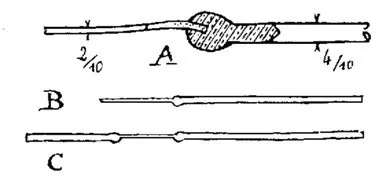
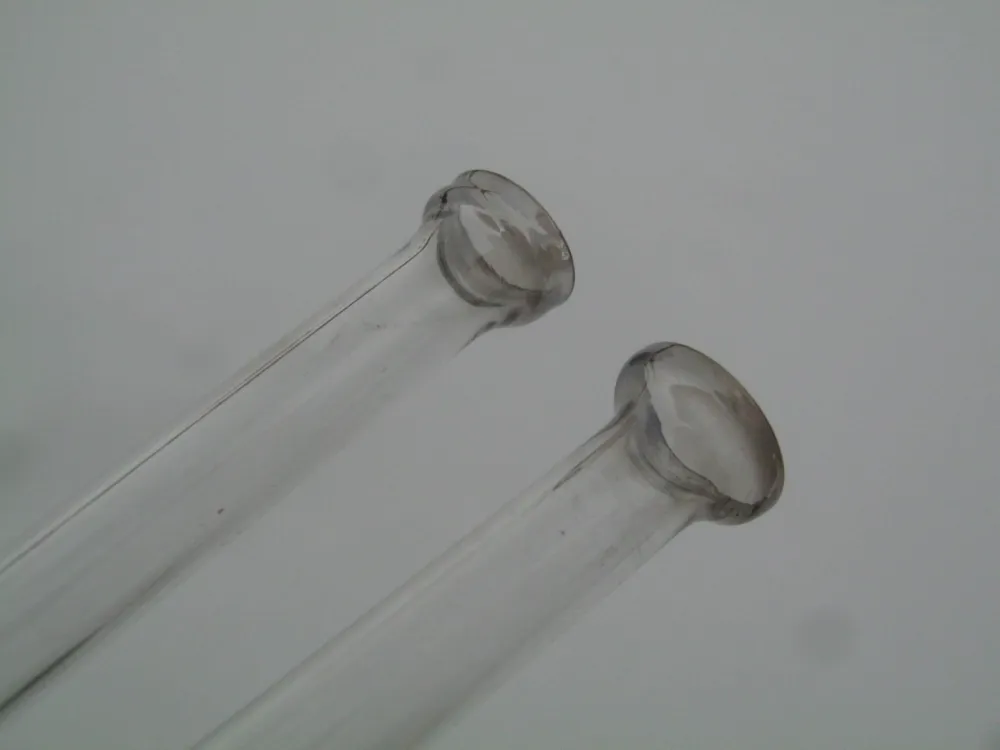
Glass: Of course! Where can you find it? At chemical product sellers. The main thing is to go there yourself, with a caliper, and choose from the offered tubes of the same quality (glass composition). Your future works will break less the better this recipe is followed. To identify the type of glass, direct the tube to the light and look at it from the end — its color varies from one type to another. We need glass of a pleasant green water color (look at the photorecord on the edge or at a broken window)(platinum group glass), its melt is colorless or close to it.
Some special glasses are pink (laboratory glass), others are purple (pre-war German X-ray tubes), etc. Leave those. Your own practice and advice will teach you to distinguish the best ones.
Glass wall thickness: 1 mm, both for small and large. Beware of the vendor who offers you much greater thickness. They cannot fit and will break (crack in flames) too eagerly during work. It is very good if these are test tubes of the right size. Take one of different diameters 8, 5, 4, and 3 mm — they will make you a good student.
2. Tools, Equipment
The glass melts in the flame of the burner. Not being a privileged person, Mr. Minye did not have a gas pipeline in his workshop, but a bottle of liquid fuel and some plumbing work solved this problem.
2. 1. Tabletop gasoline soldering lamp
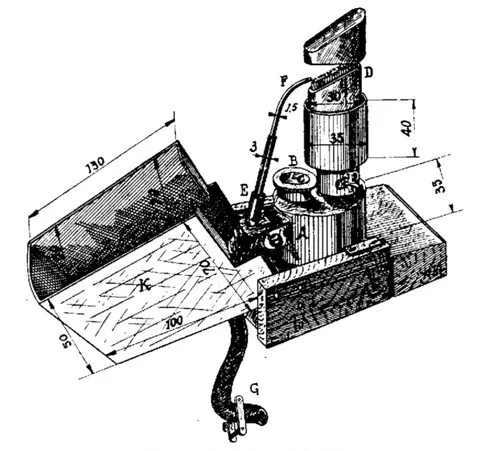
The fuel tank A, which holds about 200 ml of gasoline, has a filling neck B with a threaded or fitted cap (plug), pierced with a thin (~1 mm) hole for air intake. The burner D, welded into the tank, is flattened at the top and has a slanted — with a 1/3 incline — working cut. The burner is loosely filled with a wick that descends to the bottom of the fuel tank A. The burner D is surrounded by a water jacket for cooling, otherwise, heating during operation would result in unstable flames that tend to elongate and overheat the soldering lamp. The water jacket at its lower part has a drain valve for releasing water without tilting the lamp. A flexible rubber hose from the blower ends in an air tube E, hinged to the lamp. A composite bent nozzle F with a ~1 mm channel is inserted into the tube. The airflow is regulated by a Hoffman valve or another suitable clamp. The entire assembled lamp must be securely fixed to a dense heavy board-base to reduce the likelihood of tipping over, while the retractable metal cover K will protect the flame from drafts, operator breath, and reduce harmful glare.
2. 2. Blower
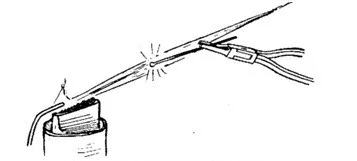
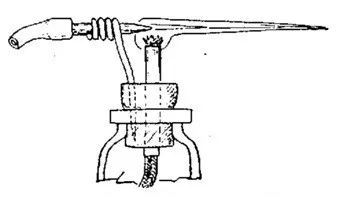
The need for quick and precise heating (sealing of the EVU) of a thin technological tube of the lamp — the stem, after evacuation. It is convenient to have a handheld (mobile) burner.
3. Summary
The ancestor-craftsmen were wonderfully inventive and undemanding, skillfully assembling quite complex works with the simplest, literally underfoot tools.
Metal soldering to glass is a complex and responsible component of any electro-vacuum work, the requirements for which are greatly simplified here due to the use of platinum — a metal very convenient due to the simplicity of preparation, reliability of the solder, and the ability to work with a wide range of different types of glass. Probably, due to this simplicity, Mr. Minye did not carefully select the glass for work — only by the color of the edges, although there exists (or existed?) a simple hot method for precise determination of glass compatibility [5]. Platinum also allowed making remarkably simple and compact, heat-resistant connections with copper through fusion.
Mr. Minye mistakenly ignored platinite — it is much, much cheaper than platinum, very easy and pleasant to work with, and can even be obtained from old factory lamp solderings by gently dismantling them with thermal shock. Platinite requires a slightly more thoughtful approach to selecting glass for work and, unfortunately, will not allow such liberties when making multi-lead soldering connections.
Fevka burners — in general, not a great rarity: working with soldering tubes in kerosene lamp flames is even mentioned in specialized glassblowing literature [6] from 1952. However, they are not recommended for use — very fire hazardous. A more convenient option for using liquid fuel is to obtain its vaporos, for example, by bubbling in a special carburetor device [7].
Mercury for a vacuum pump. Allowed to reach a vacuum of 10^-3…10^-4 Torr with a relatively simple method (dropper-piston pumps). Unfortunately, mercury is highly toxic, its circulation is now limited, and possessing a significant amount of Hg without a special license is illegal, and there is no full replacement for it. Fortunately, such residual pressure can now be easily achieved with a rotary-vane vacuum pump, for example, with a relatively inexpensive version for refrigeration and air conditioning work.
To be continued.


![Photo 1.5. Mr. Minier uses not thin aluminum wire for discharge tube electrodes (Geissler tube) for coarse indication of vacuum levels in his low vacuum system. An interesting way to connect aluminum to another metal wire is similar to fusing platinum into copper. The photo shows the author's Geissler tube with similar electrodes [1]](https://cdn.tekkix.com/imgs/2026/02/habrcom/content/312c98f286bc644a4ea062a9b19a.webp)
![Fig. 1.6. Inserting a copper wire into the molten end of aluminum is not so easy — Al metal is fluid. This is how D. Strong suggests performing such an operation [2]: 1 — tungsten (for fusing into Pyrex glass); 2 — nickel (two-pin input); 3 — strip of copper foil wound and secured onto Al; 4 — Al electrode for discharge tube. After fusion, the copper foil is removed. 5 — finished solder](https://cdn.tekkix.com/imgs/2026/02/habrcom/content/adac0ce4e116ce4cf10e99851e18.webp)
![Photo 1.7. The color of the ends of tubes made of different types of glass [3]. Green and bluish — that is the platinum group glass, honey-yellow — molybdenum, light yellow — lead glass](https://cdn.tekkix.com/imgs/2026/02/habrcom/content/beda33614007f5b2c0b290507b54.webp)
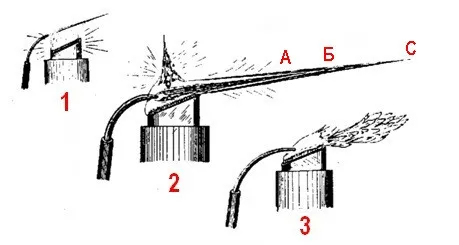
![Photo 2.3. Makeshift soldering lamp-fewka based on a kerosene lamp, filled with vaseline oil [4]](https://cdn.tekkix.com/imgs/2026/02/habrcom/content/a8eae0c230025da09825c206053a.webp)





Write comment